Superaerophobic electrode boosts alkaline water electrolysis at industrially relevant conditions
18 January 2023
Alkaline water electrolysis (AWE) is among the most developed technologies for green hydrogen generation. Despite the tremendous achievements in boosting the catalytic activity of the electrode, the operating current density of modern water electrolyzers is lower than the emerging approaches such as the proton-exchange membrane water electrolysis (PEMWE). Yet alkaline water electrolysers have one important advantage: they can operate with electrodes made of cheap and abundant materials.
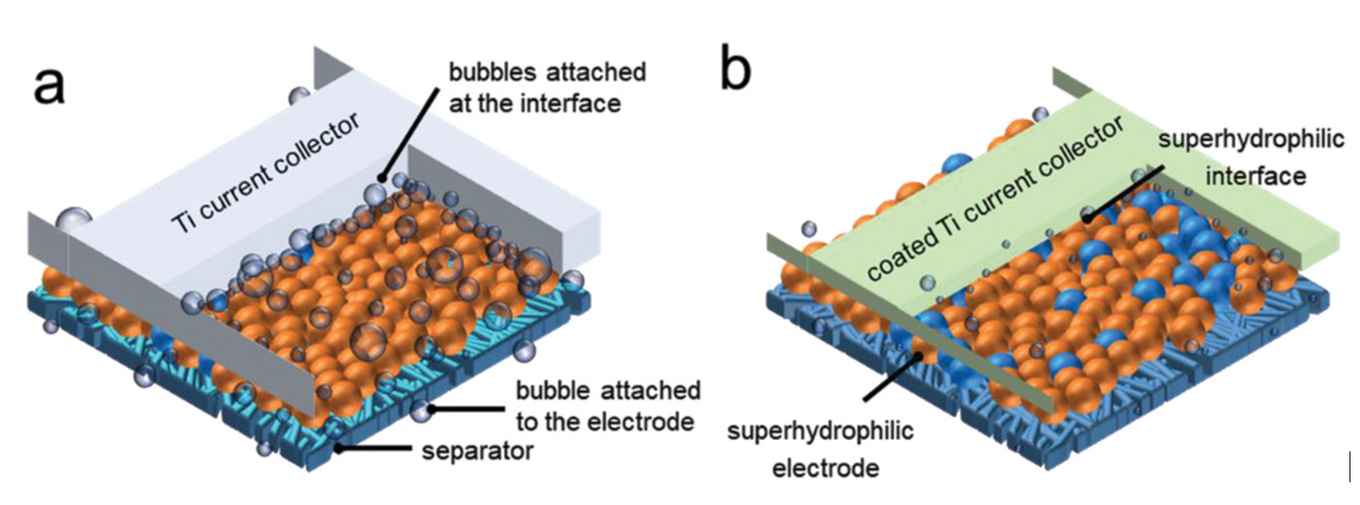
One of the dominant hindering factors are gas bubbles adhering to the electrode surface. These increase the required overpotential and introduce substantial ohmic losses. To counter these effects, various engineering strategies have been developed, resulting in electrolyzer designs incorporating flow channels, ultrasound, or magnetic fields.
Superaerophobic electrode catalysts
A more facile approach is the use of a so-called superaerophobic electrode surface that, through a clever use of materials, promotes the detachment of gas bubbles. Using such electrodes avoids changing the architecture of the conventional AWE. Although much progress has been made on developing active and superaerophobic electrode catalysts, it is still a challenge to achieve sufficiently high underwater bubble contact angles that result in rapid bubble detachment and minimal overpotential losses. What’s more, the majority of novel superaerophobic electrode materials have not been tested at high reaction rates at industrially relevant conditions.
In the paper in Advanced Science, researchers at the University of Amsterdam’s Van ‘t Hoff Institute for Molecular Sciences led by Prof. Gadi Rothenberg together with researchers at Wuhan University led by Dr Ning Yan report the development of an effective superaerophobic electrode assembly. It consists of a cobalt-nickel phosphide/oxide heterostructure that results in a superaerophobic-superhydrophilic electrode that is bifunctionally active for both hydrogen evolution (HER) and oxygen evolution reactions (OER). This electrode material supports the complete wetting of water droplets with very short spreading times and leads to underwater bubble repelling featuring 180° contact angles. The electrode assembly was tested in a realistic AWE design where it displayed high current densities at significantly reduced overpotential. This approach thus reveals the significance of bubble management over the performance of AWE, offering a promising solution toward high-rate water electrolysis.
Paper details
Lingjiao Li, Petrus C. M. Laan, Xiaoyu Yan, Xiaojuan Cao, Martijn J. Mekkering, Kai Zhao, Le Ke, Xiaoyi Jiang, Xiaoyu Wu, Lijun Li, Longjian Xue, Zhiping Wang, Gadi Rothenberg, Ning Yan: High-Rate Alkaline Water Electrolysis at Industrially Relevant Conditions Enabled by Superaerophobic Electrode Assembly. Adv. Sci., 2023, 202206180. DOI: 10.1002/advs.202206180