Evidence for the catalytic properties of ultramarine pigment
Blue paint pigment could catalyse its own ‘disease’
2 juni 2020
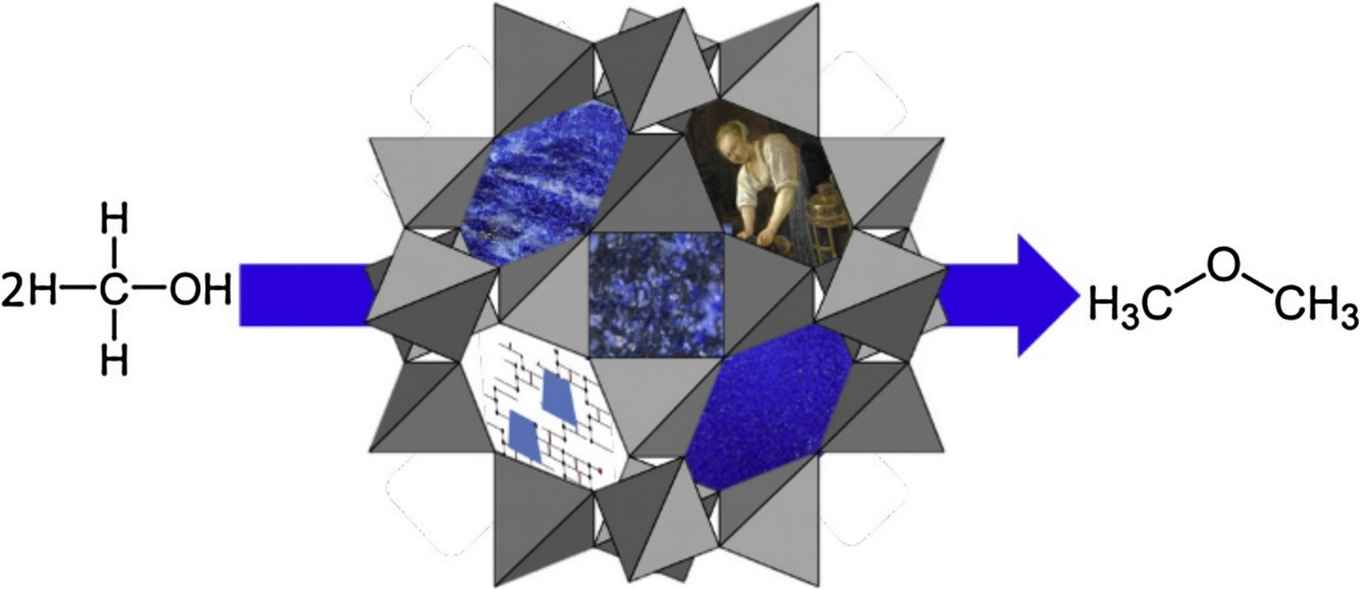
In many historical paintings containing ultramarine, the so-called ‘ultramarine disease' can be observed. It renders the blue sections of a painting dull and grey. Over the past years, researchers at the Rijksmuseum and the University of Amsterdam have studied the costly historical pigment in depth. Just recently this resulted in a Science Advances paper on the historical preparation method of the pigment from Lapis lazuli rock. Now, in an even more recent paper in the Journal of Cultural Heritage, they report with chemical detail on the catalytic activity of the pigment.
Zeolite structure
To research leader Katrien Keune this activity did not come as a surprise since the zeolite structure of the pigment resembles that of many commercial catalysts. Her team managed to confirm this in experiments on the dehydration of methanol to dimethyl ether, where the pigment showed a twelvefold increase in conversion rate when compared to a quartz sand control. The researchers also established that natural ultramarine pigment was 4.5 times more catalytically active than its synthetic counterpart. Keune thinks this is because natural lazurite-derived material contains more calcium cations than synthetic ultramarine, which are known to enhance the conversion of ethanol to diethyl ether. She suggests that the oil binder in the ultramarine paint could be degraded and oxidised due to the Brønsted and Lewis acid sites on the pigment’s cage structure.
Publication details
Kokkie Schnetz, Alessa A. Gambardella, Roe lvan Elsas, Joost Rosier, Edgar E. Steenwinkel, Arie Wallert, Pieter D. Iedema, Katrien Keune: Evidence for the catalytic properties of ultramarine pigment. Journal of Cultural Heritage, in press, available online 12 May 2020. DOI: 10.1016/j.culher.2020.04.002
See also
Chemistry World: Luxury blue paint pigment catalyses its own ‘disease’.
Nature Briefing: How paintings got the blues — and lost them.