Dutch-American experiment indicates existence of two kinds of supercooled water
Special antifreeze keeps water liquid at extremely low temperatures
8 March 2018
Water behaves completely different from most other liquids. Normal liquids shrink when cooled down, but below 4 degrees Celsius water expands upon cooling. Similar anomalies occur in the specific heat, the compressibility and many other properties of water. Below zero degrees Celsius these deviations from normal-liquid behaviour become very large.
One might think water freezes at zero degrees, but that is not always the case. In order to freeze, water molecules have to start forming a crystal, and in very clean water this process is extremely unlikely to happen spontaneously. As a consequence, very clean water can be cooled down to about minus 40 degrees Celsius without crystallizing (melting on the other hand occurs always at zero degrees). Liquid water below zero degrees is called supercooled water. YouTube provides plenty of videos showing how to make supercooled water at home and do simple experiments with it.
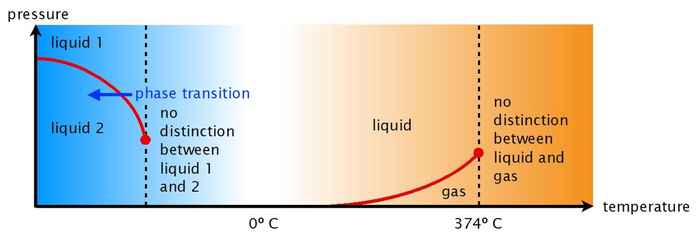
Two liquid states
In 1992 theoretical physicists from Boston University suggested that the strange properties of water might be related to the existence of two different states (a compact and a less-compact one) of supercooled water at very low temperatures and high pressure. Of course, at normal temperatures only one state of liquid water exists. Hence, according to the theory the distinction between the two liquid states vanishes at a certain temperature far below zero degrees. The disappearance of the distinction between the two liquid states above this temperature (the so-called critical temperature) would explain the anomalous properties of water at higher temperatures.
Perhaps not surprisingly, there was some controversy about this idea. At school we learn that every substance occurs in three states: solid (the molecules stacked in a regular array), liquid (the molecules randomly moving about and interacting with each other), and gas (the molecules at a large distance from each other and moving completely independently). The possibility of two different liquid states is not intuitively obvious, and water would be an exception to the rule.
In recent years, computer simulations and experiments have provided more evidence for this idea, but actually observing the predicted two liquid states in an experiment remained elusive. At first sight that would indeed seem impossible: the predicted temperature at which the two liquid states interconvert is predicted to lie far below minus 40 degrees Celsius, so water would always freeze before the second liquid state would be observable.
Antifreeze and infrared light
By using a special type of antifreeze, researchers from the UvA and ASU have now succeeded in observing a change of state in liquid water at an extremely low temperature (about 80 degrees below zero). The experiments were performed by Michiel Hilbers and Sander Woutersen (Amsterdam) and Zuo-Feng Zhao and Austen Angell (Arizona). To probe the change in liquid structure, the researchers used infrared light to measure the vibrations of the OH bonds of water molecules. The frequencies of these vibrations are very sensitive to the local liquid structure.
Initially, the Amsterdam group used glycerol as antifreeze. This substance mixes well with water and so prevents crystallization. Unfortunately, at very low temperatures (just when things got exciting) this antifreeze became ineffective: at those temperatures glycerol and water no longer mix well, and the liquid changed into a slush puppie of small ice crystals and glycerol. That seemed to be the end of the research.
Visit to Amsterdam
The breakthrough occurred when Woutersen met with Austen Angell from Arizona State University, a worldwide expert on supercooled water. In the summer of 2016 Angell, who had been on sabbatical at the UvA in 1971, was over in Amsterdam for a short visit to the UvA at the invitation of Daniel Bonn (head of the Van der Waals-Zeeman Institute of the UvA).
Angell suggested to use a very special kind of antifreeze (hydrazinium trifluoracetate), which consists of molecules that fit perfectly into the hydrogen-bond network of water, as he and his co-worker Zuo-Feng Zhao could demonstrate experimentally. Computer simulations by Bernd Ensing (UvA) confirmed this idea: these antifreeze molecules hardly change the local surroundings of the water molecules, but do prevent crystallization, even at very low temperatures.
Observation of a structural change
This finally opened up the low-temperature region. And lo and behold: during cooling, at about 80 degrees below zero, the researchers observed a sharp change in the liquid structure, accompanied by a release of heat (similar to the well-known heat release during the liquid-solid transition, but now during a liquid-liquid transition). And upon reheating the liquid, the initial liquid structure was recovered, at the same transition temperature.
With that result it appears that after 25 years, the two-liquid hypothesis has been confirmed experimentally. ‘Appears!’ Woutersen emphasizes, ‘because, mind you, this is water with added antifreeze; it’s an antifreeze that hardly perturbs the water structure, but still. And one more thing I cannot stress enough: at normal temperatures (even below zero) there is only one kind of liquid water!’
Publication details
Sander Woutersen, Michiel Hilbers, Bernd Ensing, Zhaofeng Zhao & Austen Angell, A liquid-liquid transition in supercooled aqueous solution related to the HDA-LDA transition, Science 09 Mar 2018, Vol 359, Issue 6380 pp. 1127. DOI:10.1126/science.aao7049