Experimental validation of a reaction network model for drying of fatty acid esters
10 April 2024
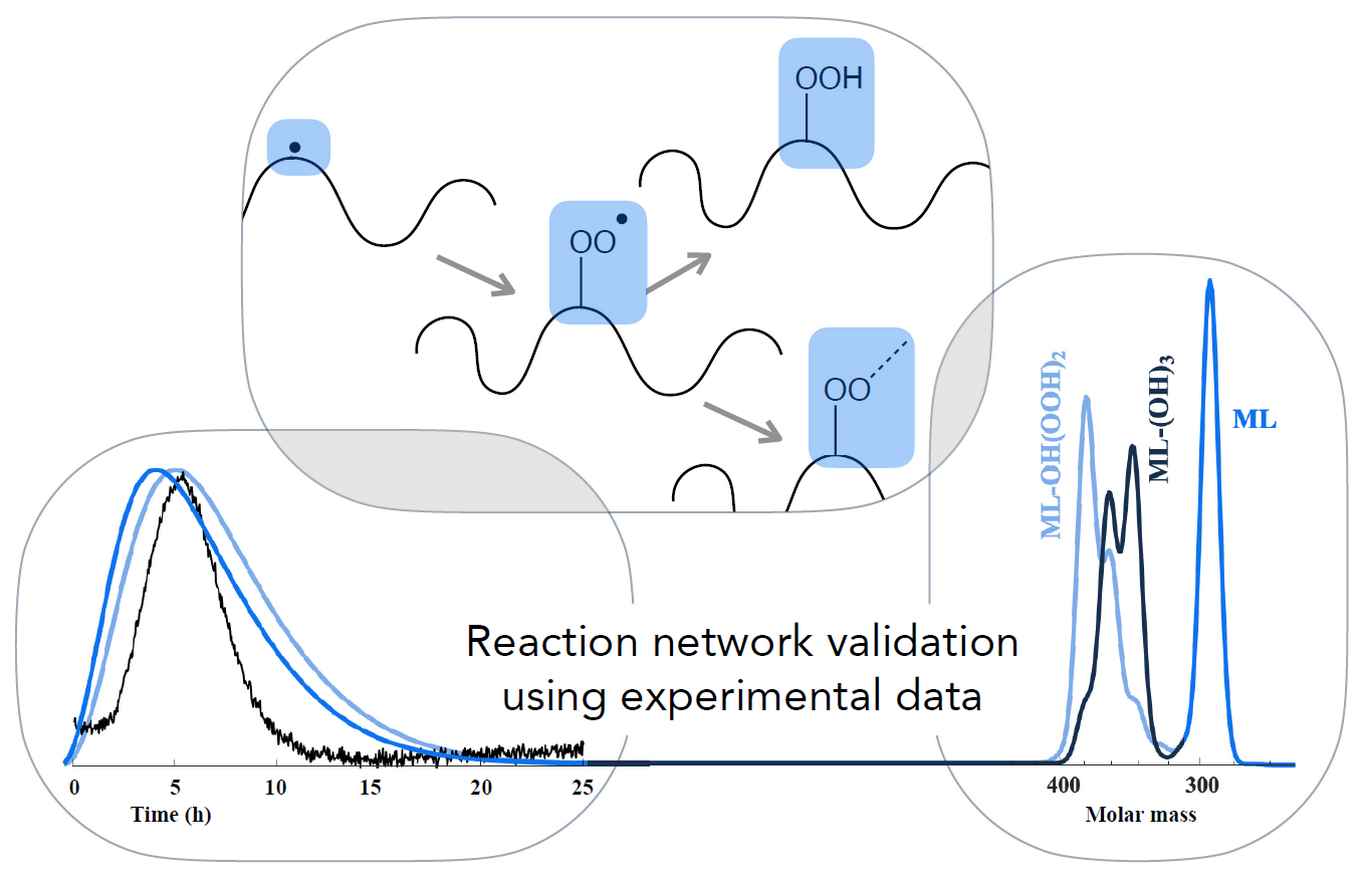
The main purpose of the work is to develop a comprehensive and validated model that describes reactions involved in drying of oil paint. It is based on recent advancements in computational modelling and combines a monomer approach with automated reaction network generation (ARNG) and random graph theory, converging towards the polymer network of the dried paint. In the current paper, the researchers show that the model reproduces experimental data of the drying process obtained with Fourier transform infrared (FTIR) and size exclusion chromatography (SEC). High intensity peaks in electron spray ionization mass spectrometry (ESI-MS) were also predicted by the model.
Abstract of the paper
A deterministic mathematical model is used to study complex chemistry and determine kinetic parameters of the autoxidation polymerization (drying) of fatty acid esters such as ethyl and methyl linoleate (EL and ML). The model was developed previously and is based on an algorithm that generates a reaction network and the associated kinetic equations in an automated way (automatic reaction network generation – ARNG). The ARNG model computes the concentrations of the monomeric species, including the number and type of crosslinks (peroxyl, ether, alkyl) they possess.
In this study, kinetic parameters of the main reaction families were estimated by adjusting them to obtain a good fit with new experimental data of various nature. Among these were: Fourier transform infrared (FTIR) spectra, electron spray ionization mass spectrometry (ESI-MS) data of EL with TiO2 cured at 70 °C and size exclusion chromatography (SEC) data of ML cured at 80 °C. Sensitivity analysis revealed that addition, bis-allylic hydrogen abstraction by peroxyl radical and peroxyl radical recombination are the most influential reactions in the considered set of functional groups. These sensitivity results indicated which reaction families were considered in the parameter estimation procedure.
Rate coefficients of key reactions like hydrogen abstraction, O2 to radical addition, OO- and O-radical addition to conjugated double bonds and β-scission could be well be determined from EL-conversion and conjugated double bond profiles from FTIR. Some tens of dominant peaks in the ESI-MS spectrum were also found in the ARNG model, while vice versa most dominant model predicted peaks were confirmed by ESI-MS. ARNG allowed detailed chemical pathway analysis in terms of intermediate products, which was helpful in elucidating the pathway for the most important acidic monomer, hexanoic acid.
Finally, the most dominant crosslink type formed turned out to be peroxyl, less ether, while alkyl is practically absent. The ARNG model of linoleate drying has thus considerably gained power to predict properties relevant to the chemical structure of EL polymer.
Paper details
Tamika E. van't Hoff, Rebecca E. Harmon, Yuliia Orlova, Joen J. Hermans, Alessa Gambardella, Piet D. Iedema: Experimental validation of a reaction network model for autoxidation of linoleate esters. Progress in Organic Coatings, Volume 189, 2024, 108363 DOI: 10.1016/j.porgcoat.2024.108363
See also
Research group Computational Polymer Chemistry.