Amsterdam chemistry students excel at national student symposium
13 maart 2018

The PAC-symposium is the Dutch national chemistry student symposium organized annually by 4 student associations: ACD (University of Amsterdam), CDL (Leiden University), USS Proton (Utrecht University) and VCSVU (Vrije Universiteit Amsterdam). Returning element at the symposium is the competition for the Young KNCV poster prize, issued by the Royal Netherlands Chemical Society (KNCV). This year the line-up for the prizes awarded by the jury was all-Amsterdam: Wowa Stroek received the first prize and € 500,-, Stephan Falcao Ferreira came in second which earned him € 250,-, and at third place Brendan Horst received a check of € 150,-.
Water oxidation for electricity storage
In the winning poster Wowa Stroek presented his research into improving the best currently known iron based catalyst for water oxidation. It was carried out under the supervision of Joeri Hessels and Prof. Joost Reek of the research group Homogeneous, Supramolecular and Bio-inspired Catalysis. This work is very relevant to the development of efficient and cost-effective large scale storage systems for renewable electricity, based on hydrogen generation by means of water splitting. Stroek modified the water oxidation catalyst reported earlier by Ma et al., hoping to substantially increase the turnover number (TON) of just 2380.
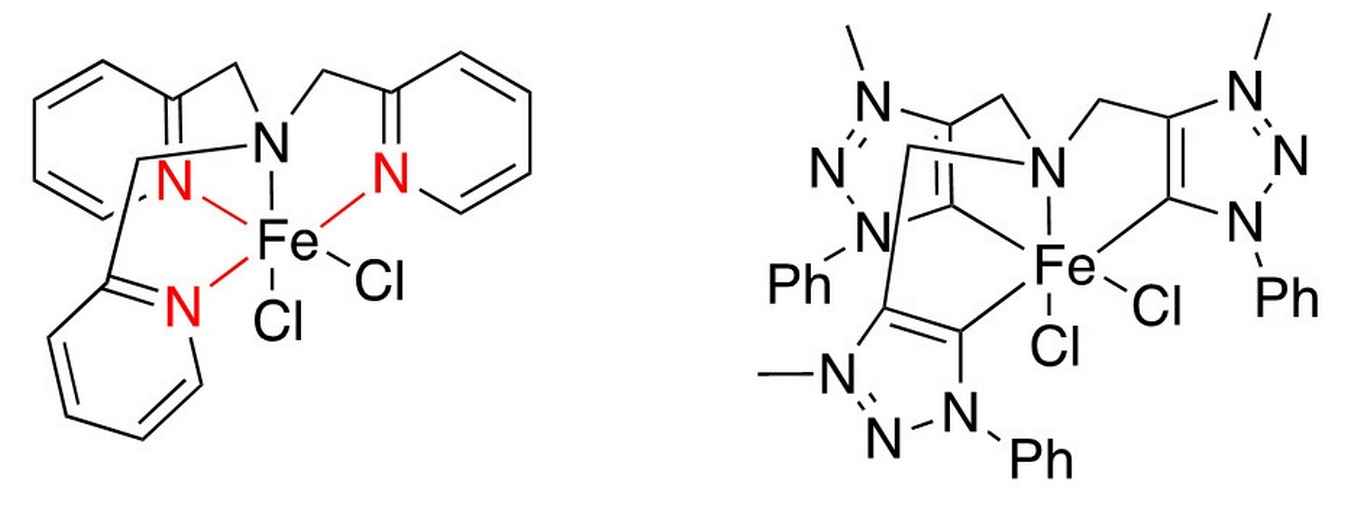
A weak point about the catalyst presented by Ma et al. (at left, as presented in Chem. Commun., 2014, 50, 12779-12782) is the coordinated pyridine, depicted in red. This is easily protonated, leading to de-coordination from the iron centre and thus destroying the catalyst. Wowa Stroek replaced the pyridine ring by triazolium rings that coordinate as mesoionic carbenes, which bind significantly stronger, do not get protonated, and are significantly more electron donating (at right).
Selective hydroformylation of natural fatty acids
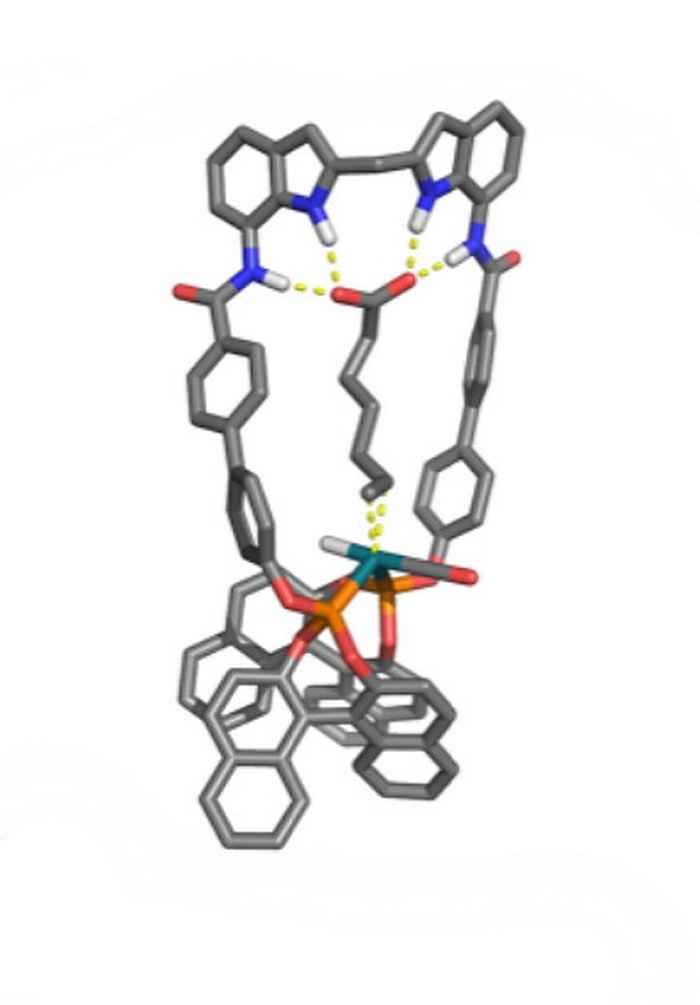
Stephan Falcao Ferreira also performed his research at the research group Homogeneous, Supramolecular and Bio-inspired Catalysis, under supervision of Pim Linnenbank. His study contributes to the development of the selective hydroformylation of natural unsaturated fatty acids that possess a double bond at the 9 position. Previously, the research group reported a neutral anion-receptor functionalized bisphosphate ligand (DIMPhos) that is able to preorganize unsaturated carboxylic acids with respect to the metal centre and thereby control the selectivity in the hydroformylation reaction. It was established that conversion and selectivity is best for those substrates in which the distance between carboxylic acid and double bond precisely spans the distance between receptor and catalytic centre. Stephan Falcao Ferreira designed a ligand with an increased distance between the anion receptor and catalytic centre, with the aim to perform selective hydroformylation of the aforementioned natural unsaturated fatty acids.
Total synthesis of govaniadine
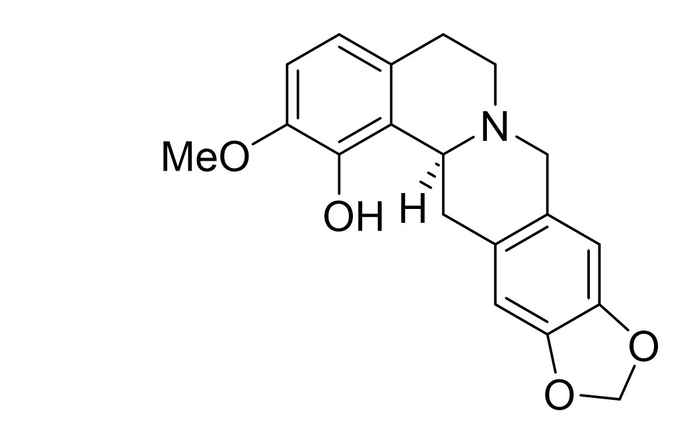
Brendan Horst performed his research at the research group Synthetic Organic Chemistry supervised by Martin Wanner and Prof. Henk Hiemstra. He worked on the regio- and stereoselective total synthesis of (S)-govaniadine. As a natural product first isolated from Corydalis govaniana Wall. in 2013, govaniadine shows interesting pharmacological properties as an analgesic (painkiller) and leishmanicide (a drug against a certain type of parasite).
Total synthesis of govaniadine suffers from substitution on the tetrahydroprotoberberine (THPB) core. However, by varying the solvent in which the tetrahydroisoquinoline ring system is formed via a Pictet-Spengler reaction, Brendan Horst achieved a selectivity in the position of the hydroxyl group up to 80%. Furthermore, using a chiral auxiliary in this step can provide a ratio of (separable) diastereomers of 4:1, making the synthesis of (S)-govaniadine possible in 99% ee.