Scientists warn: when restoring historical paintings, be careful with polar solvents
22 February 2023
Paintings conservators use solvents such as alcohol and acetone, for example to remove old varnish layers and dirt. They carry out this kind of restoration work with care to cause as little change as possible to works of art. But the commonly used polar organic solvents often contain small amounts of water. The Amsterdam researchers now show that the solvent allows the traces of water to penetrate very quickly and deeply into paint layers. There, they accelerate the formation of metal soap crystals.
Metal soaps are a well-known problem in paintings conservation. They can often be seen as small white dots on the paint surface. Moreover, they can reduce the mechanical stability of historical oil paints, which can cause them to crack and flake. "Metal soaps form naturally over time due to the chemical dynamics between the components of the paint," says first author Dr Joen Hermans. "But some paintings are a lot worse off than others. We now think that these differences are partly caused by previous treatments. That is why in our publication we warn to be on the alert for traces of water in the solvents."
Paint flakes under the microscope
The researchers established the effect of the solvent-water combination in two ways. In the laboratory, they studied 'fake paints': polymeric model systems containing zinc soaps that are on the verge of crystallising. "Using infrared light, we saw how the water penetrated deep into the paint and catalysed the crystallisation of the zinc soaps there," says Hermans. He explains that the idea for this research had been around for a long time, but he only recently managed to create the right 'metastable' model paint. After demonstrating the effect in the lab, the researchers also took a closer look at real historical oil paintings. They studied small paint chips from paintings where the pigment zinc white had been applied. There too, they found that zinc soap crystals could rapidly form when exposed to small amounts of water.
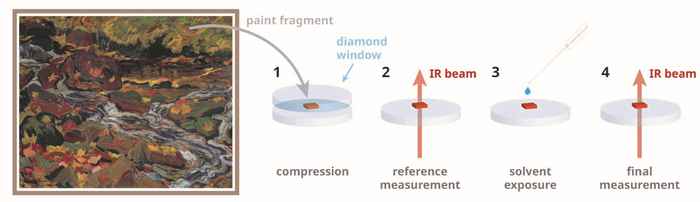
"Not all pieces of oil paint reacted in the same way, which shows that there is a huge variety in the chemical properties of oil paint," Hermans says. What did become clear, he says, is that oil paint swelling under the influence of the solvent has no influence on the zinc soap crystallisation process. "That swelling was always seen as the main cause, but we have now managed to debunk that," he says. Hermans is pleased that there is now a spectroscopic method to determine whether a specific historical paint has a risk of zinc soap crystallisation under the influence of water: “Conservators are always looking for tests to help them estimate the risks of proposed restoration treatments. Our method can give them confidence that a treatment is not going to cause harm.” For restoration and conservation practice, there is also other good news: "Short-term exposure to solvent in itself does not need to be a problem, as long as it is completely water-free."
Paper details
J. Hermans, K. Helwig, S. Woutersen and K. Keune: Traces of water catalyze zinc soap crystallization in solvent-exposed oil paint, Phys. Chem. Chem. Phys. 2023, 25, 5701-5709. DOI: 10.1039/D2CP04861B.
See also:
Research website Joen Hermans